June 18th 2024
ATiO Award Winner 2024: ZuriMed
Janick Eglauf
Scroll down

Introduction:
Emerged from the Laboratory for Orthopaedic Biomechanics at ETH Zurich and the University of Zurich, the Swiss startup ZuriMED Technologies AG was founded in 2015. Since then, the innovative company has striven to revolutionize the surgical repair of soft tissues by developing breakthrough high-potential medical devices. From the beginning, ZuriMED’s ultimate goal has always been to develop emerging technologies and products with the potential to significantly improve surgeons’ quality of treatment and ultimately patients’ quality of life. By combining established and novel biomaterials with internally developed technologies, the startup aims to bridge the gap between current surgical procedures and unmet clinical needs.
Since its founding in 2015, the startup has successfully filed more than 70 patents covering novel surgical devices, regenerative biomaterials, and technologies for soft tissue surgery. This clearly demonstrates the company’s innovative work, significant research advancements, and dedication to developing breakthrough medical devices. Furthermore, the startup secured more than 20 million USD in funding, including substantial grants from Innosuisse, the Swiss-based agency for innovation, enabling their R&D advancements and the development of a high-impact product portfolio.
To date, ZuriMED has successfully developed and out-licensed two products: the Bone-Tendon-Bone (BTB)-Converter™ for advanced hamstring graft fixation and the VariLoop™, enabling knotless ligament fixation technology. The company’s current focus is on developing and marketing the FiberLocker® System, a breakthrough technology for augmenting rotator cuff (RC) tendon repairs.
Team and Unique Location:

Dr. Xiang Li and Elias Bachmann founded the startup nine years ago, and since then, the team has steadily grown to its current size of 19 employees. The young team comprises entrepreneurs, engineers, and researchers with significant experience in their respective fields and expertise from the industry and academia. The company’s location in the heart of Zurich, surrounded by world-leading universities, grants the startup rapid access to high-quality labs and testing facilities, fostering collaborations with exceptionally specialized research groups. Furthermore, ZuriMED is based within the Swiss health cluster, including greatly renowned clinics such as the University Hospital Balgrist. Close cooperation with its physicians and researchers from the associated Balgrist Campus allows for direct feedback from orthopaedic surgeons and ensures a swift translation from development to clinical application.
This unique exchange enables the startup to understand the problems and limits of current surgical techniques in soft tissue repair and solve them by developing novel and innovative technologies. Direct feedback from world-leading physicians during these processes is indispensable and facilitates the transition of research into impactful medical solutions.
FiberLocker® System for Rotator Cuff Repair Augmentation:
Following the successful out-licensing of their first two products, ZuriMED developed a third breakthrough device, the FiberLocker® System for the arthroscopic reinforcement of rotator cuff (RC) repairs, and is currently focused on gaining access to the US market.
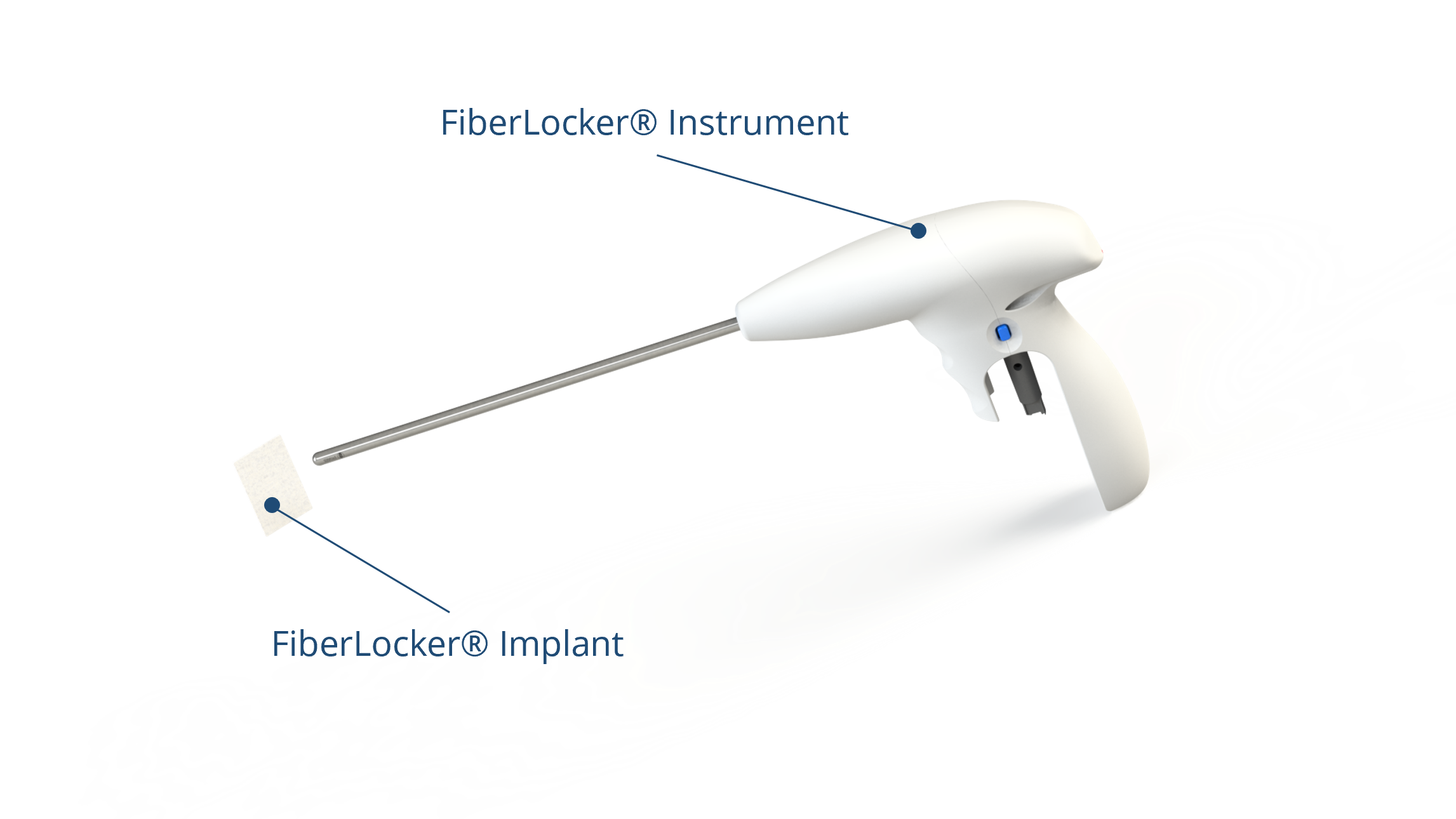
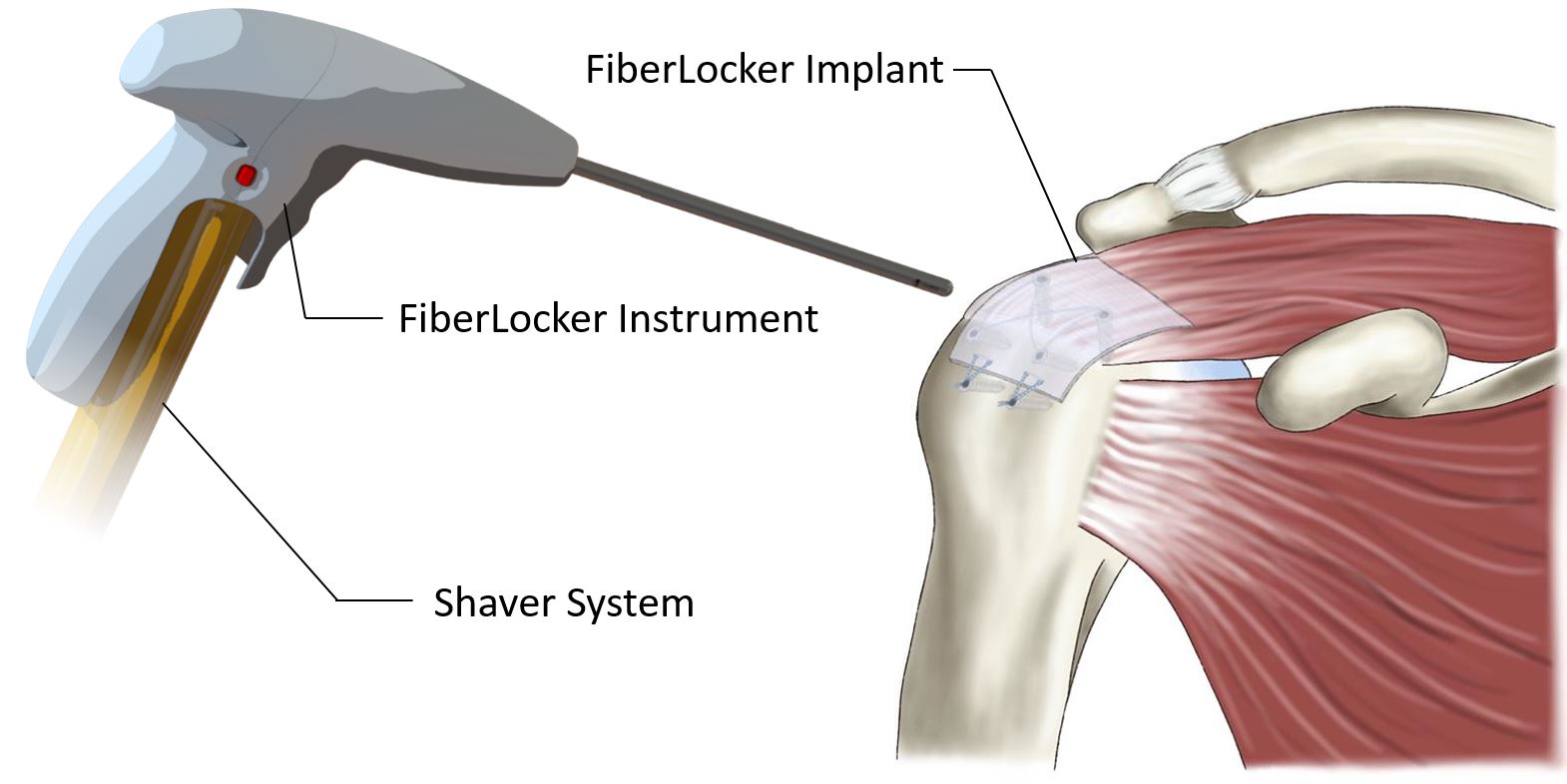
The novel system comprises an implantable patch and an instrument for its fixation on top of the supraspinatus tendon in the shoulder. As part of the RC, this tendon plays a crucial role in the stabilization and movement of the shoulder joint, and its tear represents the most common RC injury, with average prevalences described between 20.4% and 34%. Presently, the minimally invasive arthroscopic repair of the RC using bone anchors and sutures has become the gold standard. However, studies have reported repair failure rates of up to 90% after an initial RC surgery, primarily due to an imbalance in force distribution and subsequent suture slicing through the soft tissue of the tendon. The current lack of a sufficiently strong mechanical repair for RC tears presents a substantial clinical challenge, as patients often require revision surgeries following an initial failure.
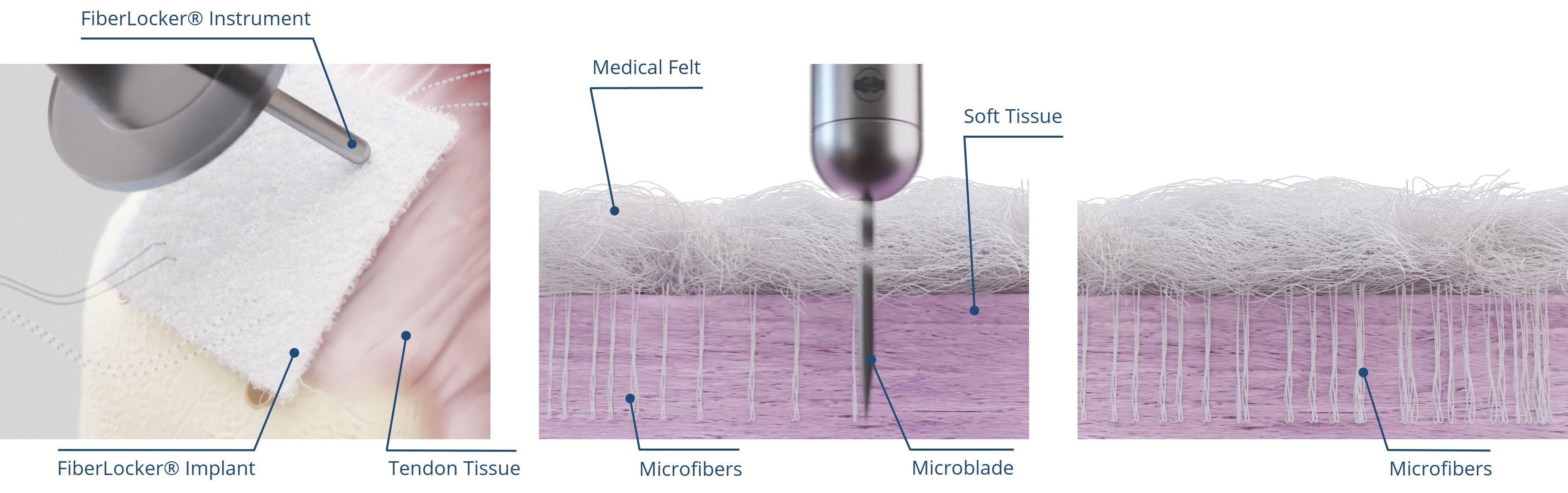
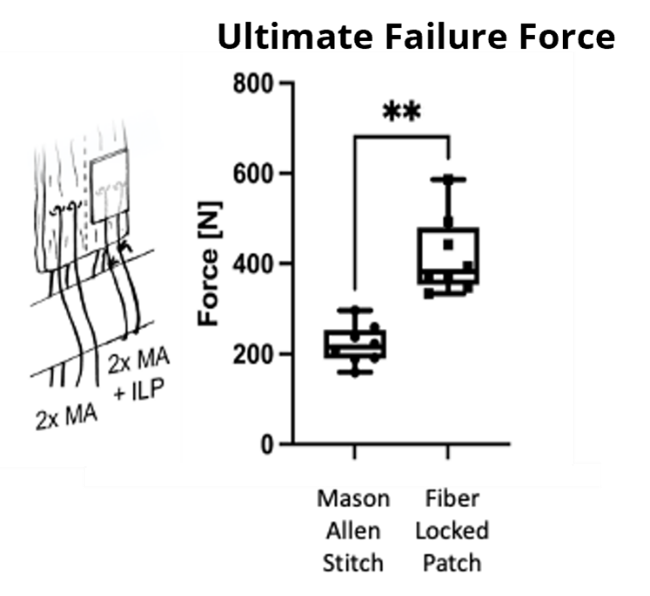
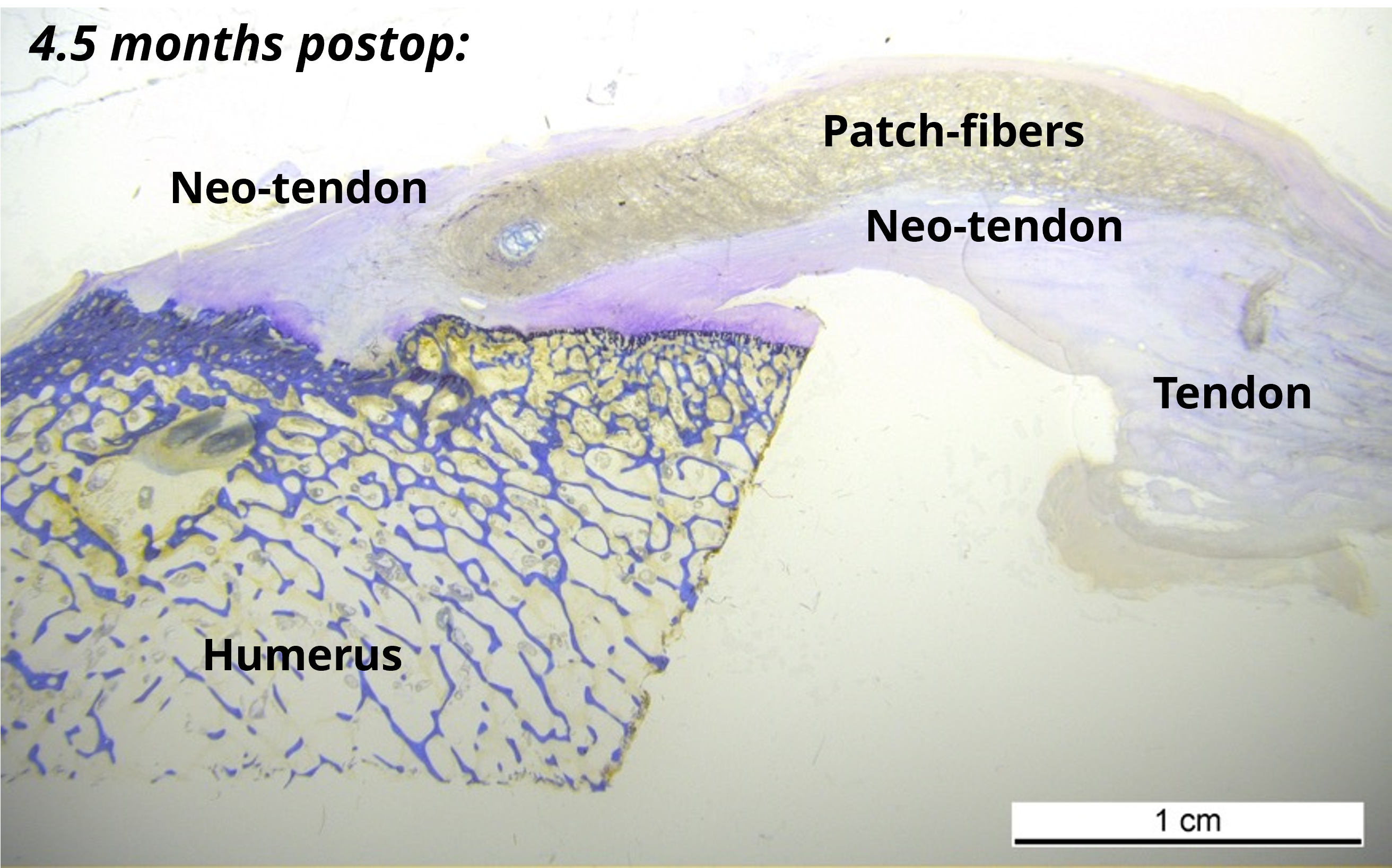
ZuriMED’s FiberLocker® System presents a promising solution, as the implanted patch serves as a reinforcement of the underlying suture repair. The implant is made of non-woven polyethylene terephthalate (PET) fibers, and the instrument, which is powered by a commercial shaver unit available in each operating room, enables the attachment to soft tissue. During the surgical procedure, the reciprocally moving microblade of the instrument pushes individual patch fibers into the underlying tendon tissue utilizing a sewing-like mechanism. Thereby, a strong interconnected network between the soft tissue and the implant is created. Uniquely, the implant can be attached to the soft tissue without the usage of additional sutures and within a short surgical time of 90 seconds. Ex vivo tests using an ovine model revealed significantly increased mechanical forces of a tendon repair when augmented with the FiberLocker® System compared to a non-augmented repair. The increased pull-out forces provided by ZuriMED’s novel system display the great potential of the technology as a reinforcement of RC repairs and a promising solution to overcome the high retear rates by providing high mechanical support from the outset.
An additional pre-clinical in vivo sheep study, conducted together with the University of Zurich, demonstrated the biocompatibility of the novel implant, as no adverse side effects were observed. Moreover, the animal study revealed excellent integration of the implant into the regenerated tendon tissue, paving the way for its translation to clinical applications.
Outlook and Next Steps:
The FiberLocker® System is not available for sale in the United States (US) or in Europe. ZuriMED’s team is currently working on securing FDA approval for their novel product and preparing the market entry in the US, planned for the first quarter of 2025. Concurrently, preparations are in progress for a pioneering first-in-human study in collaboration with the University Hospital Balgrist in Switzerland, with the expectation that the FiberLocker® System will provide its inaugural benefits to patients by the end of the year.
Furthermore, the startup is actively exploring further clinical needs in soft tissue repair and is dedicated to developing more medical devices aimed at improving surgical procedures in orthopaedics, cardiovascular medicine, and wound care to ultimately improve patients’ quality of life.
Visit their website and LinkedIn page to stay updated on future progress and developments of the innovative Swiss startup.
